
Anodising
Anodising is an electrolytic passivation process. Anodising is done through positive and negative charge, the cathode's material is transferred to the anode creating a thicker oxide layer on the surface of the metal. This is so that it has increased corrosion resistance and wear resistance, and provides a better surface adhesive for paint primers.
The positive electrode called the anode is the material that is to be treated as it attracts all of the wanted material, and the cathode, the negative electrode attracts all of the unwanted material. The thickness of the oxide layer is determined by the amount of time you leave the anode in there for and the current per unit area.
Electro-plating
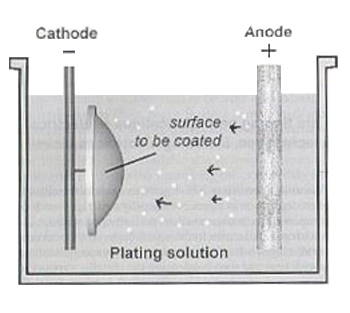 Electrolysis is used to electroplate objects. This is useful for coating a cheap metal with a more expensive one, such as copper or silver.
Electrolysis is used to electroplate objects. This is useful for coating a cheap metal with a more expensive one, such as copper or silver.The negative electrode should be the object that you want to be electroplated.
The positive electrode should be the metal that you want to coat the object with
The electrolyte should be the solution of the coating metal, such as its metal nitrate or sulfate.
The object to be plated is connected to the negative terminal of the power supply, A piece of copper is connected to the positive terminal. The electrolyte is copper sulfate solution